What is Spectrophotometer?
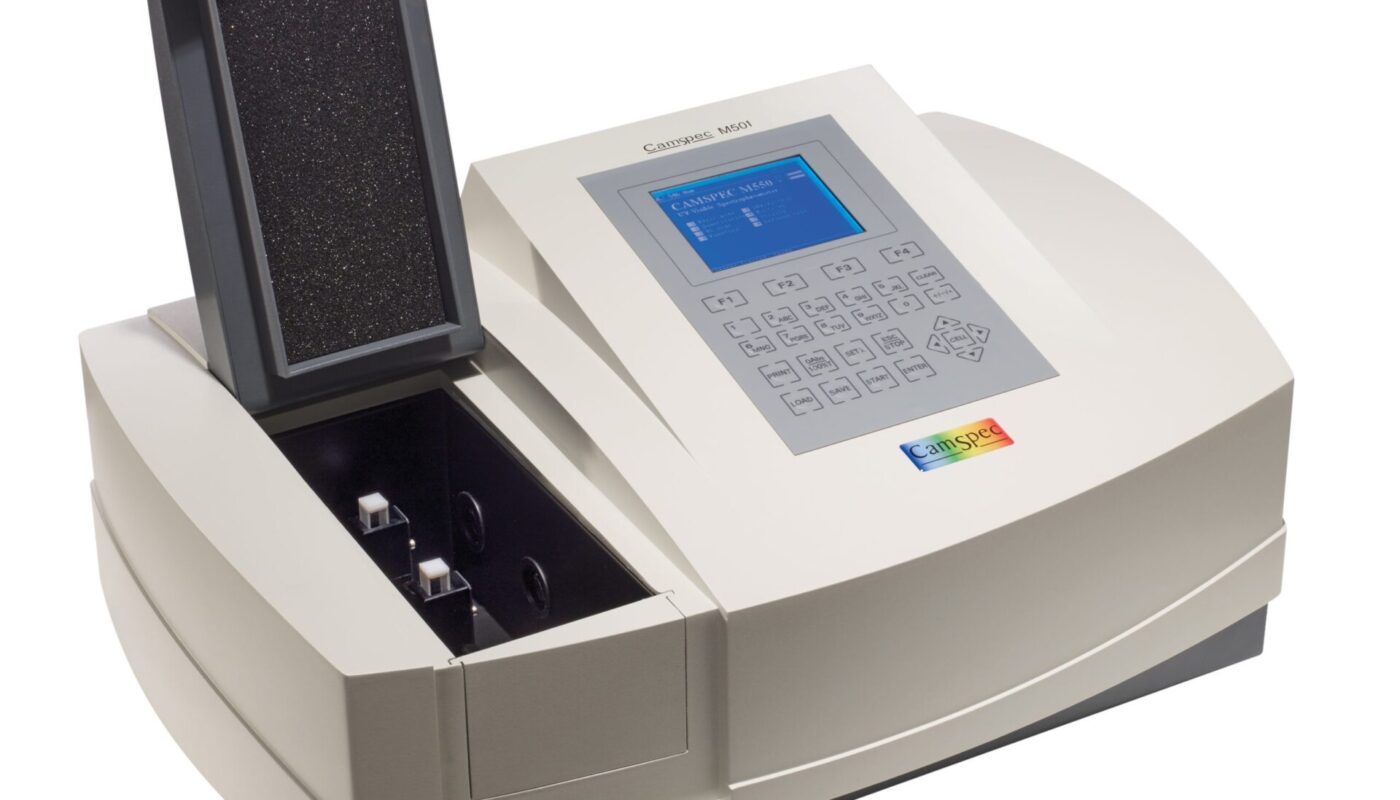
A spectrophotometer is a scientific instrument that is used to measure how much light is absorbed by a sample or transmitted through a sample. It quantitatively describes the behaviour of matter in interaction with light. Spectrophotometry is commonly used in analytical chemistry for the quantitative determination of various compounds present in a sample.
Working Principle of Spectrophotometer
A Spectrophotometer works on the principle of spectrophotometry. In spectrophotometry, a beam of light of known wavelength is allowed to pass through a sample. The intensity of the light beam before and after it passes through the sample is measured. The ratio of intensity of the transmitted light to the intensity of the incident light gives the transmittance. Absorption is calculated from transmittance.
The key components of a Spectrophotometer are:
– Light Source: Common light sources used are tungsten lamp, deuterium arc lamp, xenon arc lamp etc. These produce broadband radiation across UV, Visible and Near IR regions.
– Monochromator: It is used to select the desired wavelength from the broad spectrum emitted by the light source. It allows only a narrow wavelength range to pass through. Common types are diffraction grating and prism monochromators.
– Sample compartment: This holds the sample holder or cuvette containing the sample. It allows the monochromatic light to pass through the sample.
– Detector: Photodiode, photomultiplier tube or charge-coupled device are commonly used detectors. They detect the intensity of light transmitted through the sample and convert it into an electrical signal.
– Microprocessor: It processes the signal from the detector and displays the results as absorption, transmittance or concentration values.
Types of Spectrophotometers
Depending on the design and application, some common types of Spectrophotometer are:
UV-Vis Spectrophotometer: Measures absorption in both UV and visible regions (190-800 nm). Used for quantitative analysis of various inorganic and organic compounds.
Infrared Spectrophotometer: Analyzes molecular structure by measuring absorption in infrared region (2.5-25 μm). Used for qualitative analysis of organic compounds.
Fluorescence Spectrophotometer: Detects and measures fluorescence emitted by fluorescent compounds when irradiated by light. Used widely in biochemistry and molecular biology.
Atomic Absorption Spectrophotometer: Analyzes metals and metalloids present in samples by measuring absorption of ground state atoms. Highly sensitive and selective for elemental analysis.
NIR Spectrophotometer: Analyzes samples by measuring absorption in near infrared region between 750-2500 nm. Used for non-destructive analysis of agricultural and food products.
Applications of Spectrophotometer
Some major application areas of spectrophotometry are:
Quantitative Analysis: Spectrophotometry is used extensively for quantitative determination of different compounds present in samples. Examples include analyzing concentration of metal ions, drugs in pharmaceutical samples, dyes, etc.
Qualitative Analysis: Spectra obtained can be used to identify different functional groups present and determine molecular structure. Infrared spectrophotometry is commonly used for this purpose.
Biochemical Analysis: Absorption studies of nucleic acids, proteins, enzymes etc. provide valuable information in biochemistry and molecular biology. Used to study DNA-protein interactions, enzyme kinetics, etc.
Environmental Analysis: Spectrophotometric techniques help monitor pollutants level in air, water and soil qualitatively as well as quantitatively. Help assess environmental impact.
Food Analysis: Compositional analysis of food samples like sugars, vitamins, fats and other components is routinely done using spectrophotometry. Helps ensure food quality and safety.
Forensic Analysis: Spectroscopic techniques aid in forensic analysis by identifying traces of drugs, poisons, explosives and other materials in samples which serve as crucial evidence.
Quality Control in Industries: Continuous process monitoring and quality control of dyes, pigments, oil products etc. is achieved through inline spectroscopy. Ensures consistent product quality.
Emerging Areas of Spectrophotometry
Some emerging trends enhancing the potential of spectrophotometry are:
Portable Spectrometers: Development of miniature battery-operated spectrometers is expanding applications to field studies. Used for on-site environmental, food and pharmaceutical analysis.
Hyperspectral Imaging: Combination of spectroscopy with imaging allows spatial mapping and identification of materials/structures. Finding use in agriculture, minerals etc.
Nanoparticle Spectroscopy: Optical properties of nanoparticles depend on their size, shape and environment. Being applied for sensing, clinical imaging and other nanotechnology areas.
Surface-Enhanced Raman Spectroscopy: Combination with nanotechnology boosts Raman signal immensely, achieving single-molecule detection. A boon for ultra-trace analysis.
Computational Spectroscopy: Theoretical modeling complements experiments by predicting fundamental spectroscopic properties and interactions. Helps interpret complex spectra.
Undoubtedly, spectrophotometry will continue to evolve as a versatile analytical technique, finding diverse applications through ongoing technological advances. With improvements in precision, sensitivity, portability and cost-effectiveness, its role in scientific research as well as industrial analysis is poised to grow steadily in the years to come.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it