Market Overview:
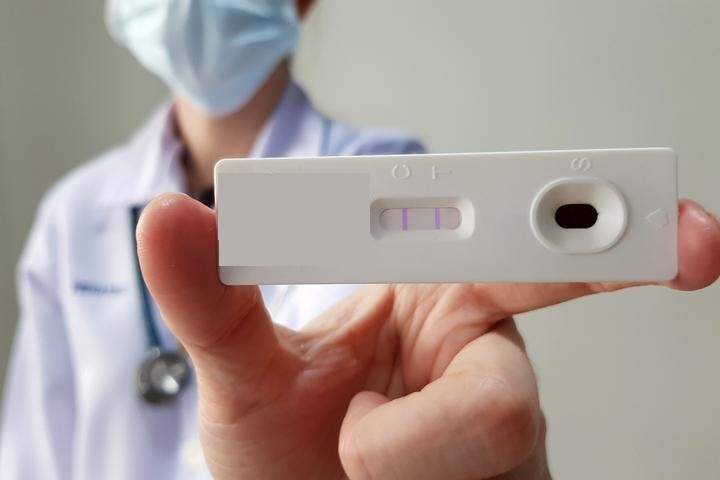
Rapid influenza diagnostic tests are point-of-care medical devices used to detect influenza viruses. These tests can provide results within 15-30 minutes directly from respiratory samples such as nasopharyngeal and nasal swabs. The growing prevalence of influenza and rising awareness about rapid diagnosis are major factors driving the market. Rapid tests help in early detection and treatment of influenza, which is important for improved clinical outcomes.
The Rapid Influenza Diagnostic Tests Market was valued at US$ 1.29 Bn in 2023 and is expected to exhibit a CAGR of 7.9% over the forecast period 2023-2030, as highlighted in a new report published by CoherentMI.
Market Dynamics:
One of the major drivers of the market is the rising demand for point-of-care testing. Rapid influenza diagnostic tests provide on-site detection of influenza virus enabling point-of-care treatment decisions. This is highly valuable in hospital emergency rooms, physician offices, assisted living facilities and nursing homes where quick diagnosis is crucial. Secondly, the market is also driven by increasing prevalence of influenza globally. As per WHO estimates, there are 1 billion cases of influenza annually worldwide with 3-5 million cases resulting in severe illness. Rapid diagnosis leads to timely administration of antiviral treatment which is important for better management of the disease.
Segment Analysis
The rapid influenza diagnostic tests market can be segmented by test type, end user, and region. Based on test type, the nucleic acid testing segment dominated the market in 2022. Nucleic acid testing provides reliable and accurate test results in less than two hours. It helps in quick diagnosis and treatment of influenza infection, which is the primary reason for its high demand.
Global Rapid Influenza Diagnostic Tests Market Segmentation
By Test Type
- Rapid Molecular Assays
- Rapid Immunoassays
- Others
By Influenza Type
- Influenza type A
- Influenza type B
- Influenza type C
- Others
By Specimen Type
- Nasopharyngeal swab
- Nasal swab
- Throat swab
- Others
By End User
- Diagnostic Laboratories
- Hospitals & Clinics
- Research Institutes
- Others
By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
PEST Analysis
Political: Governments across the globe are promoting prevention and control of infectious diseases through effective testing. Favorable regulations support the adoption of rapid influenza diagnostic tests.
Economic: The high burden of influenza on national economies due to loss of productivity necessitates investment in influenza screening. This drives the rapid influenza diagnostic tests market growth.
Social: Growing awareness about early diagnosis and treatment of influenza infection has increased the demand for point-of-care rapid tests.
Technological: Advancements in molecular diagnostic technologies enable development of rapid diagnostic tests with high sensitivity and specificity. This improves patient accessibility to influenza screening.
Key Takeaways
The global Rapid Influenza Diagnostic Tests Market Size is expected to witness high growth, exhibiting CAGR of 7.9% over the forecast period of 2023-2030, due to increasing prevalence of influenza infections worldwide. In 2023, the market size was valued at US$ 1.29 Bn.
Regional analysis: North America dominated the market accounting for maximum share in 2022 due to conducive reimbursement policies and technological advancements. Asia Pacific is anticipated to be the fastest growing region owing to rising incidence of influenza in densely populated countries and growing healthcare expenditure.
Key players: Key players operating in the rapid influenza diagnostic tests market are Quidel Corporation, Becton Dickinson, Thermo Fisher Scientific, Abbott Laboratories, F. Hoffmann-La Roche, DiaSorin, Luminex Corporation, Meridian Bioscience, GenMark Diagnostics, Sekisui Diagnostics, and Virax Biolabs. These players are focused on product launch and expansion strategies to strengthen their market presence.
Reasons to Purchase Rapid Influenza Diagnostic Tests Market Report:
1. Enhance the decision-making process by understanding the strategies that underpin commercial interest with respect to products, segmentation, and industry verticals.
2. Develop/modify business expansion plans by using substantial growth offerings in developed and emerging markets.
3. Save and reduce time carrying out entry-level research by identifying the growth, size, leading players, and segments in the Rapid Influenza Diagnostic Tests Market.
FAQ’s
Q.1 What will the market development pace of the Rapid Influenza Diagnostic Tests Market?
Q.2 What are the sales, revenue, and price analysis of the top players of the Rapid Influenza Diagnostic Tests Market?
Q.3 What are the market opportunities and threats faced by the vendors in the Rapid Influenza Diagnostic Tests Market?
*Note:
1. Source: CoherentMI, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it