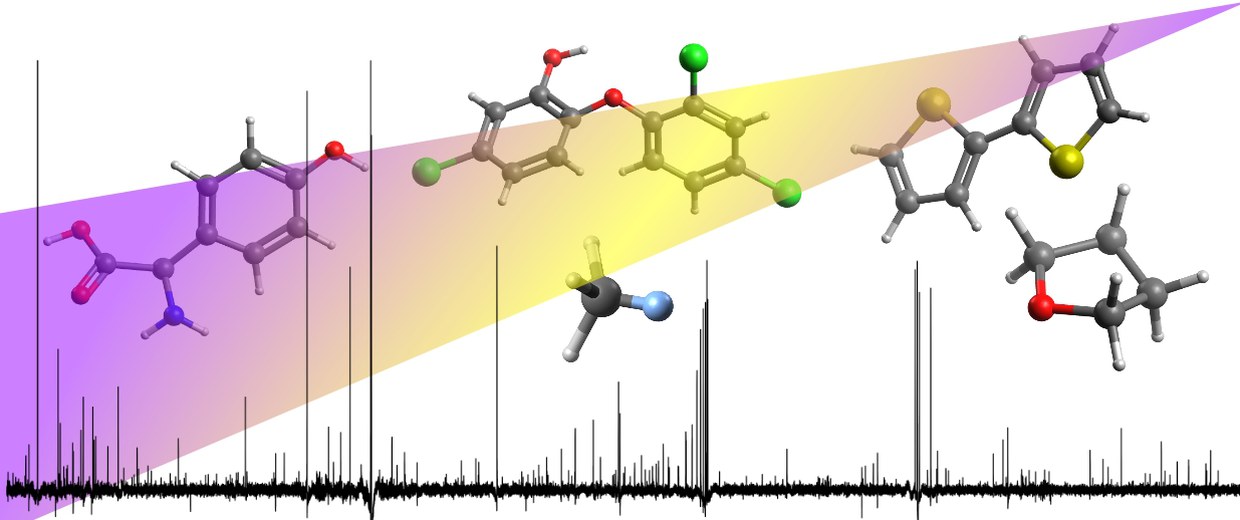
Molecular spectroscopy refers to the study of the interactions of electromagnetic radiation and molecules. When electromagnetic radiation interacts with molecules, it causes transitions between different quantum states of the molecules. Studying these molecular transitions through spectroscopy techniques provides invaluable insights into the structure, composition and dynamics of molecules.
What is Molecular Spectroscopy?
Molecular spectroscopy relies on the fact that molecules can absorb, emit or scatter specific wavelengths of electromagnetic radiation as they transition between different quantum states. Quantum states are allowed energy levels that molecules can occupy. The differences in energy between these quantum states correspond to specific wavelengths of electromagnetic radiation. When molecules absorb radiation matching these energy differences, they undergo transitions from lower to higher energy levels. Similarly, molecules in an excited state may undergo spontaneous or induced transitions to lower energy states accompanied by emission of radiation.
By studying the interaction of molecules with electromagnetic radiation across different regions of the electromagnetic spectrum from microwave, infrared, visible, ultraviolet to X-rays and gamma rays, molecular spectroscopy provides information about the allowed quantum states and transitions in molecules. Different regions of the electromagnetic spectrum correspond to transitions between different types of quantum states in molecules like vibrational, rotational, electronic and spin states. Essential properties of molecules like their structure, bonding, composition and intermolecular interactions can be determined by identifying the characteristic patterns or spectra resulting from these transitions.
Infrared Molecular Spectroscopy
One of the most important regions for Molecular Spectroscopy is the infrared region from 700 nm to 1000 μm wavelengths. Infrared spectroscopy relies on the fact that molecules undergo transitions between vibrational and rotational energy levels when interacting with infrared radiation. The resonant absorption of specific infrared frequencies by a molecule provides a ‘fingerprint’ of its chemical composition and structure.
Infrared spectroscopy is routinely used for qualitative and quantitative analysis of organic and inorganic compounds. The position and relative intensities of absorption bands in an infrared spectrum are directly related to the types of bonds and functional groups present in a molecule. For example, the presence of a carbonyl (C=O) group gives rise to a strong absorption band between 1600-1800 cm-1. Similarly, hydroxyl (O-H) and amine (N-H) groups absorb strongly at 3200-3600 cm-1 and 3100-3400 cm-1 respectively.
Characterizing Protein Structure with Vibrational Spectroscopy
Vibrational spectroscopy techniques like infrared and Raman spectroscopy are extremely useful for studying protein structure and conformational changes. Proteins consist of amino acid building blocks linked together by peptide bonds (C=O—N-H). The amide I and amide II vibrations arising from these peptide bonds are sensitive to the local protein environment and secondary structure elements like α-helices and β-sheets.
By analyzing the position and intensity variations of characteristic amide I and II bands, detailed information on secondary structure composition and unfolding/refolding mechanisms in proteins can be deduced. Vibrational spectroscopy is a label-free method for probing conformational changes in real-time under physiological conditions without the need for complicated sample preparation. This makes it a powerful tool for studying protein folding, protein-ligand interactions and enzyme mechanisms.
Ultraviolet-Visible Molecular Spectroscopy
The ultraviolet-visible region from 200-800 nm corresponds to electronic transitions in molecules. Electronic spectroscopy relies on absorption and fluorescence spectroscopic techniques to study σ → σ*, n → π* and π → π* electronic transitions in molecules. These electronic transitions are localized and sensitive probes of molecular structure.
Ultraviolet-visible absorption spectroscopy is routinely used in chemistry laboratories for quantitative and qualitative analysis. For example, Beer-Lambert law relating absorbance to concentration can be used to determine unknown concentrations. Electronic transitions are also highly sensitive to functional group substitutions and conjugation effects. This allows identification of functional groups and conjugation/aromaticity in unknown organic compounds.
Fluorescence spectroscopy finds widespread applications in biochemistry and biophysics. Many biologically important fluorophores like tryptophan, NADH, flavins undergo characteristic fluorescence upon ultraviolet or visible excitation. Protein tertiary structure, binding interactions, folding dynamics and enzyme activity can be studied by tracking fluorescence spectral changes of these intrinsic or extrinsic fluorophores. Fluorescence resonant energy transfer (FRET) is an important technique used to measure intermolecular distances at the nanometer scale.
Applications of Molecular Spectroscopy
The wide ranging applications of molecular spectroscopy arise from its ability to provide structural and dynamical information about molecules non-destructively. A few key applications are:
– Identification of unknown materials: Comparing experimental spectra with spectra databases allows identification of compounds in fields like forensics, clinical diagnosis, environmental monitoring and astrobiology.
– Protein secondary structure analysis: Infrared and CD spectroscopy are used to characterize secondary structures of proteins and monitor conformational changes.
– Drug discovery: High-throughput screening of compound libraries employs spectroscopy to identify hits for further optimization based on interactions with target proteins/receptors.
– Catalyst characterization: In situ spectroscopy is used to understand reaction mechanisms by probing surfaces and adsorbates on catalysts during reactions.
– Atmospheric chemistry: Fourier transform microwave and millimeter wave spectroscopy study molecular composition and reactions in planetary atmospheres.
– Materials science: Raman and infrared microscopy probe microstructures, defects and interfaces in materials from semiconductors to pharmaceuticals.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it