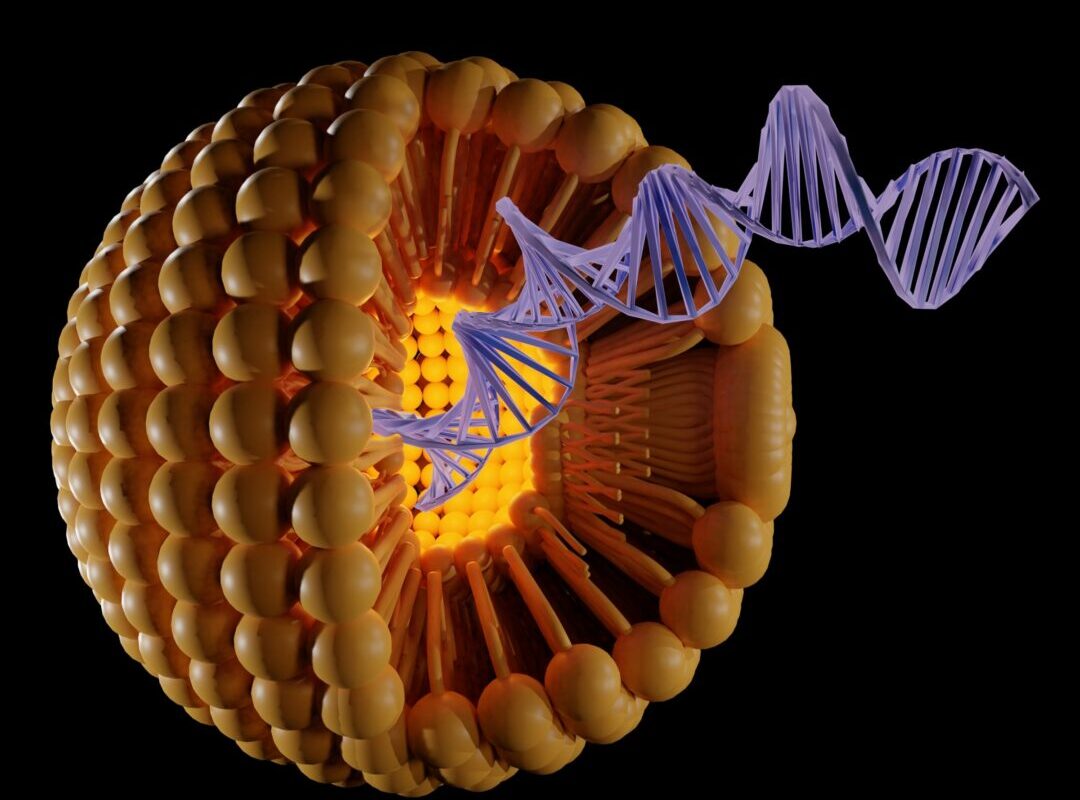
Introduction to Liposomes
Liposomes are spherical vesicles made of phospholipid bilayers that are used as carriers for drug and gene delivery. Phospholipids form an amphipathic bilayer that resembles the structure of cell membranes. By enclosing drugs within liposomes, drugs can be delivered safely to target cells and tissues in the body. Liposomes protect encapsulated drugs from premature degradation and can release drugs in a controlled manner at the target site. Over the past few decades, liposomes have emerged as one of the most promising drug delivery technologies for various biomedical applications.
Types of Liposomes
Based on the lamellarity or number of bilayers, liposomes are classified into three main types – uni-lamellar vesicles (ULV), oligo-lamellar vesicles (OLV), and multi-lamellar vesicles (MLV). Uni-lamellar vesicles contain a single phospholipid bilayer and are of uniform size, typically between 20-100 nm in diameter. Oligo-lamellar vesicles contain a few bilayers (2-5), while multi-lamellar vesicles contain multiple bilayers separated by aqueous compartments and are usually larger than 100nm. The type of liposome used depends on the characteristics and stability required for the encapsulated drug. For instance, targeted delivery requires stable uni-lamellar liposomes of defined size.
Surface Modifications of Liposomes
Liposome Drug Delivery can be modified on the surface through conjugation of ligands like antibodies, peptides, hormones, and carbohydrates to achieve active targeting. Liposomes modified with polyethylene glycol (PEG) chains exhibit increased stability or “stealth” properties due to reduced uptake by the mononuclear phagocyte system. Antibody-conjugated liposomes can undergo receptor-mediated endocytosis at target cell surfaces for tumor-specific intracellular drug delivery. Ligands are attached to liposomes through covalent bonds or non-covalent interactions and provide selective delivery to organs, tissues or cell types based on ligand-receptor binding. Surface modifications create versatile “smart” delivery systems with applications in cancer chemotherapy, immunotherapy, and diagnostics imaging.
Targeted Drug Delivery using Liposomes
Targeted drug delivery aims to increase drug concentration at diseased sites while reducing systemic side effects. Passive targeting relies on enhanced permeability and retention (EPR) effect in tumor tissues which have leaky vasculature and poor lymphatic drainage. Long-circulating “stealth” liposomes accumulate selectively in tumors, providing a platform for active targeting. For example, doxorubicin-loaded PEGylated liposomes (Doxil) demonstrated superior therapeutic efficacy with reduced heart toxicity compared to free doxorubicin. Antibody-modified liposomes achieve intracellular delivery of drugs in cancers over-expressing target receptors like HER2 and folate receptor. siRNA delivery using nucleolin-targeted liposomes holds potential for cancer gene therapy. Overall, ligand-conjugated and cell-specific liposomes improve drug management of various cancers, infections and genetic diseases.
Specialized Liposomes
Apart from conventional liposomes, research explores more specialized liposomal formulations. Thermoresponsive liposomes loaded with heat-activated drug release mechanisms when localized hyperthermia is induced in tumors through external means like ultrasound, magnetic fields or infrared irradiation. These “smart” delivery systems enhance drug penetration of solid tumors. Stimuli-sensitive liposomes incorporating pH-labile bonds undergo endosomal escape following cellular uptake, facilitating cytosolic delivery of biomacromolecular drugs otherwise vulnerable to lysosomal degradation. Exosome-mimetic liposomes designed to resemble endogenous extracellular vesicles achieve active targeting and bypass reticuloendothelial clearance for treatments requiring systemic circulation. Overall, continued developments in surface engineering and inclusion of intelligent triggers will expand clinical applications of advanced liposome systems.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it